Patients should understand the important differences between investigational treatments/procedures and approved treatments/procedures for patients with neurological conditions.
Here, to help you decide the best course of care for yourself or a loved one, we’ll dive into the distinctions between investigational and approved treatments and procedures.
We’ll also cover the importance of clinical trials to test investigational treatments, and how patients might benefit from participating in them.
What is an investigational treatment or procedure?
As the U.S. Food and Drug Administration (FDA) explains, investigational treatments and procedures are emerging therapies designed to treat a specific neurological condition. At this stage of development, the treatment or procedure lacks the necessary research backing to earn full approval for use in the general population.
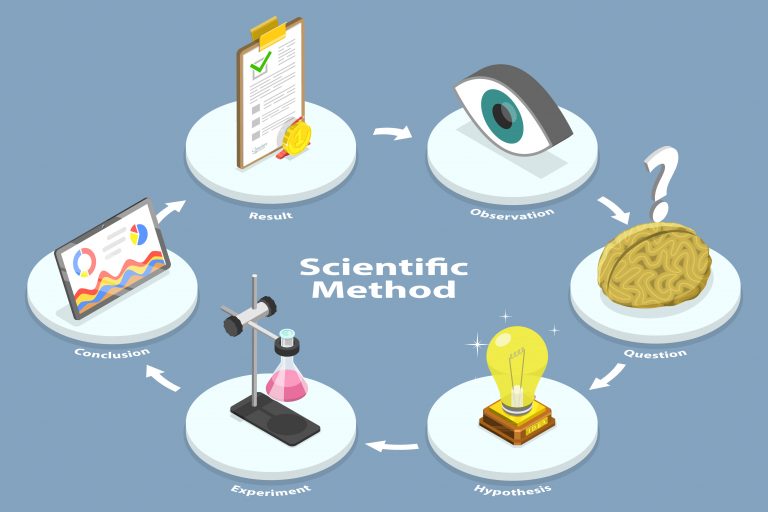
Investigational treatments are also often called “experimental treatments.”The experimental stage is a critical step in the scientific method that researchers follow to make vital healthcare discoveries and pioneer new therapies in the field of medicine.

From a regulatory perspective, the primary difference between investigational treatments/procedures and non-investigational ones is that investigational treatments have not yet earned approval from the FDA.
The FDA is the main governmental body responsible for certifying treatments and procedures for legal administration to American patients. Without FDA approval, doctors can’t use treatments in regular clinical practice for the general public, no matter how beneficial they might be.
For instance, before a new drug can be introduced to market, it must be evaluated by the FDA’s Center for Drug Evaluation and Research (CDER). To perform the evaluation, the CDER assembles a team of statisticians, physicians, pharmacologists, chemists, and other scientists to review the data from the drug manufacturer’s clinical trials that test the drug’s safety and efficacy.

We’ll discuss how clinical trials work in more detail later.
What is the purpose of investigating new treatments?
The National Institute of Neurological Disorders and Stroke identifies more than 400 known neurological conditions. Many of these have no proven treatment, and many more have limited treatment options, often with massive side effects.
These facts require that researchers continually pioneer new potential treatments to improve quality of life for patients and, in many cases, to save their lives.
The goal of investigating new treatments is to use empirical, quantitative data to prove or rule out a new medicine or procedure’s effectiveness to treat a particular health condition.
In addition to documenting a treatment’s efficacy, another goal of the investigation is to look for adverse side effects – in other words, undesired effects on patients’ health that might mean the treatment is too dangerous or harmful for clinical use.
Before a treatment can be certified as legitimate, researchers must rule out the possibility of the placebo effect. This term refers to the well-documented phenomenon of patients’ perceived or actual improvement based on their own belief in the treatment, rather than from any physiological impact of the treatment.
Institutional investigations of emerging treatments or procedures in humans are called clinical trials.
Why would a patient try an investigational treatment?
The natural question arises: why would a patient ever consider trying an investigational treatment instead of one that’s already been through rigorous testing?
Here are a few potential reasons that a patient might receive an investigational treatment:
- There are currently limited existing treatments for the patient’s condition
- The patient experienced negative side effects from existing treatments
- An investigational treatment has shown great promise in the early stages of clinical testing
- As a public service, patient volunteers are needed to conduct critical research studies to test new investigational therapies; without them, breakthrough treatments could not be developed
Under the right circumstances, clinical trials can benefit both the individual patient participaticipating in them as well as society as a whole, given the important role that trial volunteers play in the development of breakthrough therapies for difficult-to-treat neurological conditions. We’ll discuss at greater length the social value of clinical trials coming up.
What are clinical trials for investigational treatments?
Clinical trials are the rigid, strict research protocols that test an investigational treatment and document its effectiveness and side effects. They also help determine the ideal dosage for the greatest efficacy with the least risk of side effects.
Clinical trials are typically privately funded by drug manufacturers (pharmaceutical firms), medical device companies and other private-sector entities, but they may also receive public funding via the National Institutes of Health (NIH).
For a more detailed explanation of how clinical trials are conducted, please see our authoritative blog post.
Researchers work hard to protect patient safety during clinical trials of investigational treatments
The terms “investigational” or “experimental” might seem scary, but patients should be aware of the extensive lengths that researchers go to in order to safeguard patient safety.
First of all, all clinical research in the United States is overseen by monitoring boards staffed by licensed doctors. Examples include Institutional Review Boards (IRB) and Data and Safety Monitoring Boards (DSMB). In addition, research is subject to oversight by the FDA throughout clinical trials.
To further minimize the risk to volunteers, clinical trials go through a series of phases. They start with very small numbers of volunteers at very small doses in the initial phases, progressing to higher doses and greater numbers of trial participants as the investigational treatment moves through the process.

Medical ethics (and the law) require that researchers obtain informed consent from patients before beginning any clinical trial. That means patients are made fully aware of all known risks as well as potential benefits of the investigational treatment based on the current state of knowledge.
To learn more about how researchers protect volunteer safety, check out our authoritative blog post on that topic.
How to enroll in a clinical trial for an investigational treatment
Clinical trials require volunteers from within the community to be successful. To benefit the whole world, you or a loved one could become a critical part of the work we do to advance and test breakthrough treatments for a variety of neurological conditions.
To browse through our ongoing work to see if one of our clinical trials might be relevant to you, please view our current NWFLCRG studies.
What are the benefits to patients of participating in a clinical trial?
Aside from contributing to the advancement of potentially life-changing treatments for patients worldwide, here are the personal, immediate benefits to clinical trial participants:
- You’ll be seen by a board-certified physician for free
- You’ll enjoy unprecedented access to potential new treatment options before they’re available to the public
- Depending on the trial, you might be paid for your time and travel
Learn more about investigational treatments and procedures
Our central mission is to develop new treatments in the field of neurology to transform patients’ lives for the better. We rely on brave volunteers from the community like you to see that mission through to fruition.
Contact Northwest Florida Clinical Research Group for more fact-based information about investigational treatments and procedures.