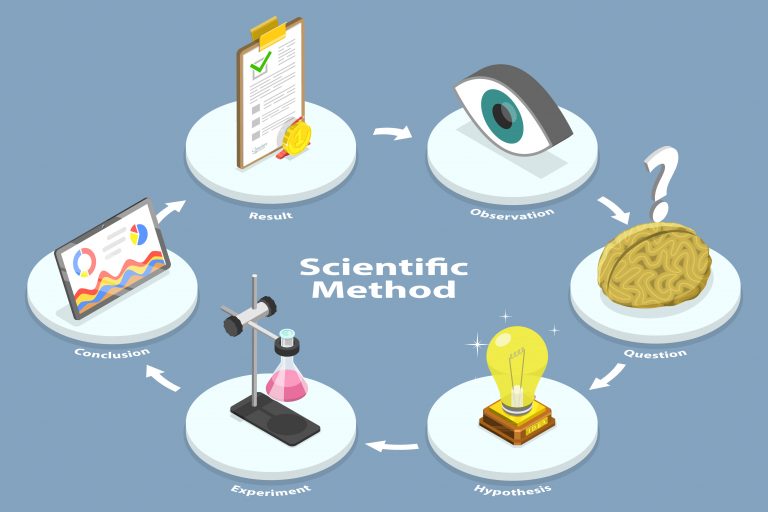
Clinical trials test proposed new methods (using a new drug or an emerging treatment modality) to address specific health conditions. These interventions may be surgical, behavioral, or medical in nature. The focus of a clinical trial can be either on treating an existing condition or preventing it altogether.
Firms developing new therapeutics must prove, through clinical trials and other vetting procedures, the safety and efficacy of their novel products before they gain approval for distribution in the United States.
Let’s get into what clinical trials are, what purpose they serve, and how they are conducted.
The Regulatory Role of the US Food and Drug Administration in Clinical Trials
Federal law in the United States requires that any new therapeutic be thoroughly tested for safety and efficacy before being introduced to market for patients to use.
The US Food and Drug Administration (FDA) is responsible for overseeing the vetting process of new therapeutics in clinical trials. Their regulators visit clinical trial sites and review data to ensure that everything is above-board.
The FDA has the ultimate authority to approve new drugs for American patients or not based on the results of clinical trials.

Clinical Trials For Determining the Efficacy of New Drugs or Treatments
The National Institutes of Health explains the main purpose of clinical trials:
“[Clinical trials] are the primary way that researchers find out if a new treatment, like a new drug or diet or medical device… is safe and effective in people.”
Research teams also compare (in phase 3 of clinical trials) the new drug or treatment to the current standard to assess how it performs against conventional treatment methods. Please see our article on the phases of clinical trials for more details about the process.
Clinical Trials For Determining the Safety of New Drugs or Treatments
Often, newly minted drugs show enormous initial promise for treating the condition they were developed to address, only to be discovered later on in the study process to have unintended, often dangerous side effects.
Most drugs carry at least some risk of some side effects; the key is to balance the risk to reward ratio and make an informed decision about whether the potential benefits outweigh the potential side effects.
That’s one of the purposes of clinical trials.
How Do Clinical Trials Help Determine the Correct Dosage of a New Medication?
Clinical trials are important for determining the ideal dosage of a given medication under study. Getting the correct dose of an emerging medication is just as important as selecting the correct medication in the first place.
Too little of an effective drug can nullify the therapeutic benefits; too much of an effective drug can have serious adverse consequences (too much of any good thing is a bad thing, as the folk saying goes).
As a simple example, acetaminophen (the active ingredient in Tylenol and other common over-the-counter drugs) can be an effective, well-tolerated medication for pain relief. In too high of dosages, though, it can lead to liver complications and potentially even death.
Accordingly, one of the most important jobs for researchers looking into newly developed drugs is to determine the right dose for prescribers. This is especially important for children, who have lower body weights and are particularly at risk of drug side effects.
Who Funds Clinical Trials?
Funding for clinical trials in the United States can come from either the public or private sector, or, often, a combination of those. The most common funders of clinical trials are:
- Pharmaceutical companies
- Medical device companies
- The National Institutes of Health
The Journal of the American Medical Association reports that, currently, the majority of funding for clinical trial research comes from pharmaceutical corporations, which have the financial means to undertake such projects:
“The pharmaceutical industry plays a vital role in financing the research required to develop new drugs. While grants from the National Institutes of Health (NIH) fund most basic research in academic laboratories, it is largely an industry that bears the cost of identifying new molecular entities and testing them in animal models and human subjects.”
How Do Researchers Recruit Clinical Trial Participants?
All clinical trial participants are volunteers. Researchers screen applicants for certain criteria to determine if they are a good fit for the study. These criteria can include:
- Having the particular condition that the new drug or treatment method is intended to address
- Good health with no underlying medical conditions
In addition to looking for specific criteria, researchers also reject applicants based on “exclusion criteria,” factors that may include gender, age, a specific disease, treatment history.
The Risks and Benefits to Trial Participants
Trial volunteers can reap benefits from trial participation but also willingly subject themselves to some degree of risk.
The potential pros of trial participation can be:
- The first beneficiaries of promising new treatments
- Their health is monitored by trained professionals
- Contributing to the development of possible transformative medical breakthroughs
The potential cons of trial participation can be:
- A certain unknown risk of side effects (especially in phases 0-2)
- Might miss out on proven conventional treatments
Ultimately, each individual (often in consultation with their family, personal doctor, or caretaker) must weigh the benefits and risks to decide on his or her involvement in a clinical trial. Researchers are obligated to disclose all relevant data to volunteers so that they can make an informed decision.

Exciting Clinical Trials in the Field of Child Neurology
The field of child neurology is rapidly advancing. Researchers are working on multiple new treatments for a variety of childhood neurological conditions.
Here is a small sample of some of the most promising areas of ongoing clinical trials in 2021-22 at different stages of clinical research:
- Enzyme replacement.
- Antisense oligonucleotides (ASOs)
- Adeno-associated viruses (AAV) to introduce genes

We diligently track the emerging research in this quickly evolving field to stay abreast of the latest developments.
Child Neurology Center of Northwest Florida
The Child Neurology Center of Northwest Florida is your local leader in expert brain and nerve care for children. Through our holistic treatment strategies and state-of-the-art equipment and techniques, we dedicate all of our extensive resources to effectively treating a wide array of childhood nervous system disorders.
We’re always available during regular business hours to book an appointment for your child or to discuss any issues related to your child’s health and wellness. Please don’t hesitate to contact us; we’re here to serve you.